Supersaturating the Solutions
Introduction
The required humidity is generated only over a saturated solution. This requires as much chemical as possible to be dissolved and there still to be solids remaining. It is also a requirement that the solution is homogeneous and saturated throughout, not stratified. So long as solids remain mixed with the solution then the equilibrium saturation is naturally self maintaining. For some chemicals it is possible to heat a solution until all the solids dissolve and then cool it again without the crystals precipitating back out as solids. When this happens the solution is supersaturated. Since the concentration is then higher than the saturation value, the humidity generated will be lower than expected. This problem is simply avoided by ensuring that some solid crystals are always present even when the solution is at its warmest. As long as there are any crystals present, the solution is unlikely to supersaturate as it cools.
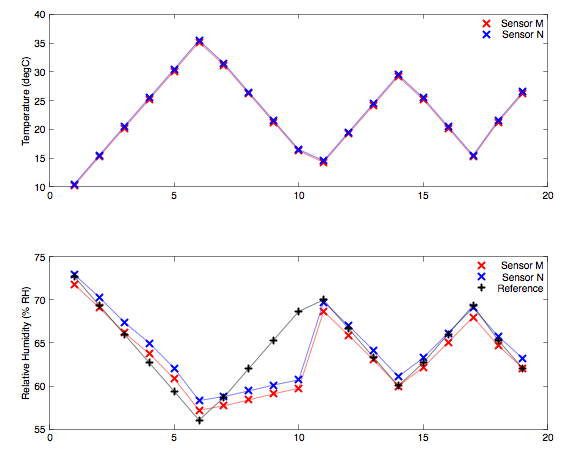
This plot for ammonium nitrate illustrates the supersaturation. The solution was stepped in temperature between 10 and 35 °C. As the temperature increases the measured humidity falls in close agreement with the expected reference. At data point six, the warmest, all the solids had dissolved. From data point seven onwards the solution is being cooled but the did not recrystallize. It supersaturated and the humidity does not retrace the expected path. At data point eleven the solution suddenly recystallised en masse and the humidity stepped back to the expected saturation value. For subsequent thermal cycles from data point eleven onwards, the temperature was not taken quite so high so that even at the warmest point the solids did not all dissolve. The solution now recrystallises in an ordered manner as the solution cools and correct saturation is maintained.
Conclusion
Do not allow the solution to supersaturate. Make sure there is always a considerable excess of solids present. It is probably best to stir the solution if you can though I have not done so in any of these experiments. If not stiring, in order to keep the solution homogeneous, the sample will need to be quite shallow so that solids break the surface allowing solid, liquid and gaseous phases all to be in contact and able to equilibrate.
If you have comments or suggestions feel free to contact me:
2017-02-24 6:33 PM